BD Medical - Pharmaceutical Systems manufactures and sells prefillable drug delivery systems to pharmaceutical and biotech companies.
Drug Delivery Systems
Committed to drug delivery excellence. Our vision is to enable customer success with high quality and innovative drug delivery solutions that improve patient well-being.

- Overview
- Products
- Services & Capabilities
- Resource Library
- News & Events
- Contact Us
Overview
Introducing Drug Delivery Systems
Innovative design, global manufacturing, product development expertise
With over 50 years of experience, we are empowering customer success through innovative drug-delivery solutions that improve patient quality of life.
At a glance…
-
>3Bn
Prefillable Syringes* (PFS) manufactured per year1
-
$2Bn
Yearly revenue (2022)**
-
7500
Associates globally
-
8
Manufacturing plants worldwide
-
Global Headquarters
in Pont-de-Claix, France
Products used by more than 500 Pharmaceutical and Biotechnology companies.2
BD customers include 27 of the top 30 pharmaceutical companies in the world.3
~70% of the top 100 pharmaceutical companies around the world rely BD Medical-Pharmaceutical Systems prefillable drug-delivery systems for a wide variety of therapeutic applications.4
BD has generated over 4000 patents that are enforced in more than 25 countries.5
Patient Well-Being
We are focusing on patient well-being
There is a patient at the end of each of the billions of drug delivery devices that we make at BD. That is why we have invested in deep knowledge around patient experience, injection science and combination product development to anticipate customer challenges, manage complexities, and reduce risks. We also invest heavily to support our commitment to quality. Through our extensive global manufacturing network, we aim to offer security of supply and business continuity to our customers.
These, plus our ability to leverage the industry and health authority partnerships of a global healthcare company, are game changing advantages that BD brings to our customers’ drug delivery challenges.
We leverage these assets as we develop innovative solutions that provide peace of mind to customers and contribute to greater patient well-being.
Solutions to meet your needs across therapeutic areas
BD Medical - Pharmaceutical Systems manufactures and sells prefillable drug delivery systems to pharmaceutical and biotech companies.
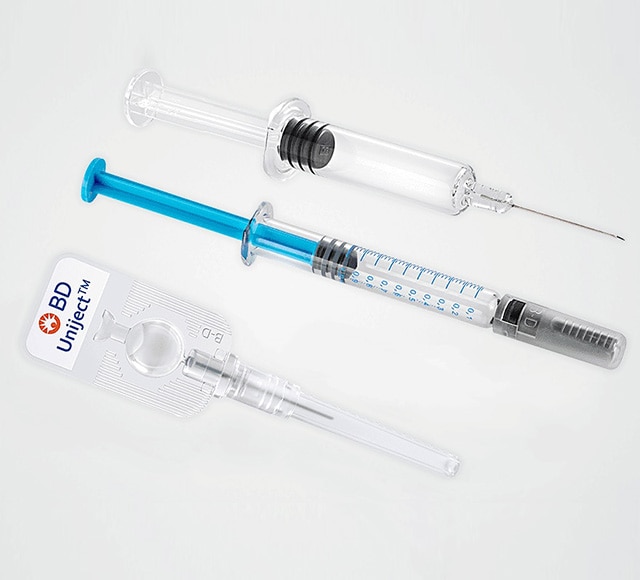
Featuring a comprehensive portfolio of innovative prefillable syringe systems, BD offers drug delivery solutions designed to help decrease development risk, improve speed to market, and increase flexibility to meet patients’ needs across a wide range of drug types and therapeutic areas.
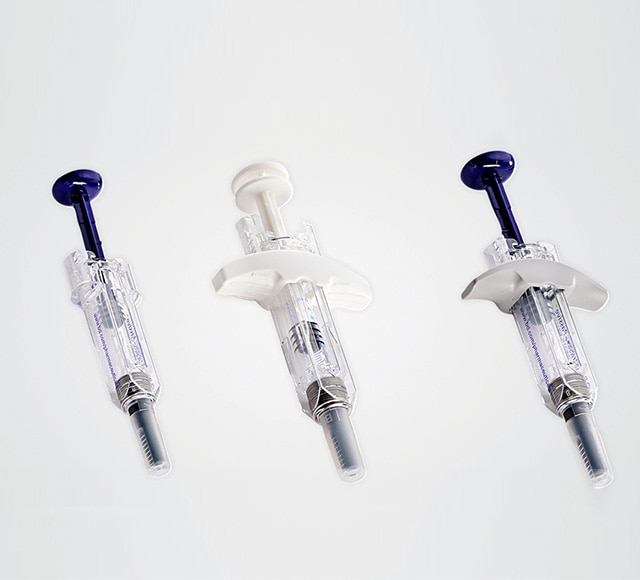
BD Safety & Shielding Systems provide pharmaceutical companies with a wide range of both active and passive manual injection solutions available in 0.5ml, 1ml, and 2.25ml formats, suitable for a variety therapeutic indications.
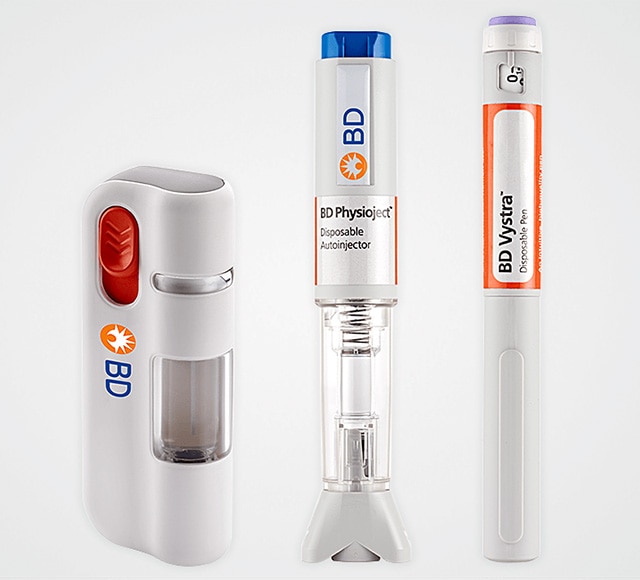
BD offers an extensive portfolio of patient centric, self-administered drug delivery solutions including disposable autoinjectors, disposable variable dose pen injectors, and wearable injectors designed to deliver a wide range of volumes and viscosities and help de-risk combination product development.

To fulfill our system approach, we provide a complete set of components for your prefillable syringe systems.
Trusted Expertise
A history of innovation and market leadership

2022
BD Effivax™ Glass Prefillable Syringe

2021
BD SCF™ PremiumCoat® Plunger Stopper
2021
BD Libertas™ Wearable Injector

2021
BD Evolve™ On-body Injector

2021
BD Neopak™ XtraFlow™ Glass Prefillable Syringe

2019
ZebraSci combination product services

2018
BD Hylok™ Glass Prefillable Syringe
2018
BD Neopak™ XSi™ Glass Prefillable Syringe
2014
BD Hypak™ for Vaccines Glass Prefillable Syringe
2013
BD UltraSafe Plus™ 1mlL Passive Needle Guard

2013
BD Sterifill Advance™ 50ml Polymer Prefillable Syringe

2013
BD Neopak™ Glass Prefillable Syringe

2012
BD acquires Safety Syringes Inc.
2012
BD Vystra™ Disposable Pen

2011
BD AutoShield Duo™ Safety Pen Needle

2010
BD Physioject™ Disposable Autoinjector
2009
BD Hypak™ for Biotech Glass Prefillable Syringe
2004
BD Hypak Physiolis™ Glass Prefillable Syringe

2003
BD Sterifill™ Polymer Prefillable Syringe
2001
BD Eclipse™ Needle Shielding System

2000
BD Accuspray™ Nasal Spray System

2000
BD SCF™ FluroTec® Plunger Stoppers
1998
5-bevel needle
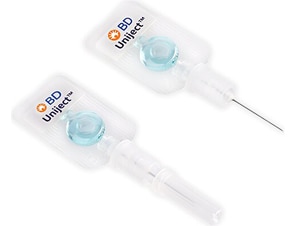
1998
BD Uniject™ Auto-Disable Prefillable Injection System

1996
BD Sterifill™ Polymer Prefillable Syringe
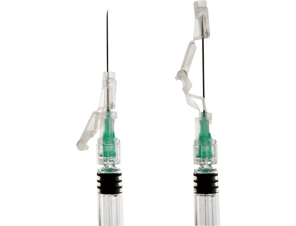
1996
BD SafetyGlide™ Needle Shielding System

1990
Polypropylene corrugated materials
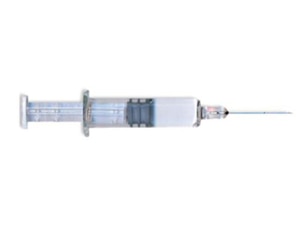
1975
First SCF Filling process

1954
BD Hypak™ Glass Disposable Syringe

2022 - Ease of Use℠ Award
BD Physioject™ - recognized by the Arthritis Foundation®

2021 – Vaccine Industry Excellence
Best Production/Process Development

2021 – Frost & Sullivan
BD Intevia™ - Best Practices Award

2020 – Derwent
Top 100 Global Innovator

2019 – Good Design® Award
BD Libertas™ wins prestigious Good Design® Award


2015 – Thomson Reuters
Top 100 Global Innovators

2015 – PDMA OCI
Outstanding Corporate Innovator

2015 – Fortune
World’s Most Admired Companies
Let’s have a conversation.
Fill out our form to have one of our experts contact you.
Notes
* PFS containers
** BD Annual report 2022
References
BDM-PS Financial file November 2022, “Actual FY22” Permiter.
BDM-PS Financial file November 2020, "Actual FY20" Permiter.
BD Customers compared to Evaluate Pharma ranking FY2019
IQVIA: IQVIA dataset extracted on March 11th, 2021, from the IQVIA Smart MIDAS' on-line platform, includes WW sales of injectable drugs. Top 100 Corporation (based on IQVIA standard units 2020) selling PFS, Autoinjectors or Pens. TA included: Acute biologics, Anticoagulants, Chronic, Small molecules, Vaccines
BDM-PS Patents – In force – Worldwide – October 2021.
Solutions to meet your needs across therapeutic areas

Featuring a comprehensive portfolio of innovative prefillable syringe systems, BD offers drug delivery solutions designed to help decrease development risk, improve speed to market, and increase flexibility to meet patients’ needs across a wide range of drug types and therapeutic areas.

BD Safety & Shielding Systems provide pharmaceutical companies with a wide range of both active and passive manual injection solutions available in 0.5ml, 1ml, and 2.25ml formats, suitable for a variety therapeutic indications.

BD offers an extensive portfolio of patient centric, self-administered drug delivery solutions including disposable autoinjectors, disposable variable dose pen injectors, and wearable injectors designed to deliver a wide range of volumes and viscosities and help de-risk combination product development.

To fulfill our system approach, we provide a complete set of components for your prefillable syringe systems.
Let’s have a conversation.
Fill out our form to have one of our experts contact you.
Services and Solutions
Services and Solutions
BD understands the drug development process and objectives at each stage and offers a wide range of services to help develop container/device strategy and life cycle management plan for drugs.

BD Pharmaceutical Services and Solutions are designed to help you achieve your combination product goals, from development to launch.

BD PartnerPath™ Program aims to support pharmaceutical organizations that value speed to market in combination product development.1
Our Capabilities
We’ve built our services and capabilities around a deep understanding of the combination product development process and the key objectives at each stage, from discovery to delivery.
We are committed to partnering for your sustained commercial success.

Effective system integration can be critical to the success of combination products for pharmaceutical companies.1

We manufacture our solutions with a strict focus on continuously advancing quality, consistency and capacity.

Quality is the foundation of customer and patient trust; it is at the core of what we do to provide safe, effective products and services. All BD associates are committed to a culture of quality inspired by the customers and patients we serve.
Let’s have a conversation.
Fill out our form to have one of our experts contact you.
References
- Market Research “Challenges with System Integration" [external study], Franklin Lakes, NJ, USA: GLG 2019
Documentation
Biologic Drugs
Hospital Treatments
Vaccines
Specialty Pharmaceuticals
View research articles published in a journal and written by experts sharing study results that offer you insight into a particular topic.
View a scientific poster, such as those used for display at BD tradeshows or seminars to provide detailed information on a research topic
Learn more about order details, product specs, and technical information by viewing a brochure
Let’s have a conversation.
Fill out our form to have one of our experts contact you.
Events
Join BD at DCAT Week 2025, from March 17-20 in New York City. Here’s what you can expect:
- BD Latest Developments
- Networking Opportunities
- BD Interactive Sessions
- BD Current Solutions
Jan 22-23 - Hall 7.2, Booth E55 - Paris Expo, Porte de Versailles
- Jan. 22 - 15:15 – 🎤 Keynote
- Jan. 22 - 15:50 – 🧪 Presentation
- Jan. 22 - 16:05 – 🗣️ Panel Discussion
- Jan. 23- 10:50 – 📊 Presentation
October 28-29
BD Presentations
- Solo Talk: October 28 from 12:15PM to 12:30PM ET
- Tech Talk
Boston, Massachusetts - USA
October 22-23
2 BD Presentations
- Tech Talk: Wednesday, October 23 from 9:25AM to 9:35AM
- Podium presentation: Wednesday, October 23 from 13:55PM to 14:15PM
Phoenix, Arizona - USA
March 26 4:45pm - 6:15pm CDT
Salon C
Hilton Chicago Hotel – Chicago, IL
California, USA
February 28 - 29, 2024
DoubleTree Hilton Anaheim Resort
Porte de Versailles
January 24-25, 2024
Hall 7.2 – BD Booth E60
-
 Sep 13, 2022
Sep 13, 2022BD Effivax™ Glass Prefillable Syringe Features Enhanced Technology that Builds on Company’s Decades of Expertise in the Prefillable Syringe Market and Supports Customers’ Manufacturing Needs FRANKLIN LAKES, N.J. (September 13, 2022) – BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today introduced a next-generation glass prefillable syringe (PFS) that sets a new standard in performance for vaccine PFS with new and tightened specifications for processability, cosmetics, contamination and integrity.1
-
 May 18, 2022
May 18, 2022Companies to work together with the aim of optimizing advanced technologies for use in prefillable syringes FRANKLIN LAKES, N.J. (May 18, 2022) – BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, and Mitsubishi Gas Chemical Company, Inc. (MGC), a R&D-oriented chemical manufacturer based in Tokyo, Japan, today announced that they have entered into an agreement to investigate further development of OXYCAPT™ - an innovation from MGC that integrates the best of plastic and glass for plastic syringes.
Let’s have a conversation.
Fill out our form to have one of our experts contact you.
Contact Us