Cybersecurity at BD

Welcome to the BD Cybersecurity Trust Center
In healthcare, cybersecurity includes more than protecting systems and data. It also includes protecting patient safety and privacy. Upholding strong cybersecurity measures and continuing to advance cybersecurity is part of our commitment to customer trust. BD works diligently to help protect the confidentiality, integrity and availability of BD products, manufacturing systems and enterprise IT. We strive to meet high security standards so our customers can focus on what matters most: caring for patients.
- About Us
- Trust Center
- Bulletins and Patches
- Disclosure Process
- Helpful Resources
2023 Product Security Annual Report
BD products and systems are designed to be secure and are developed using industry-leading cybersecurity standards, including those from ISO and NIST.
BD maintains a culture of transparency and collaboration with customers, industry stakeholders and suppliers to establish and uphold industry best practices.
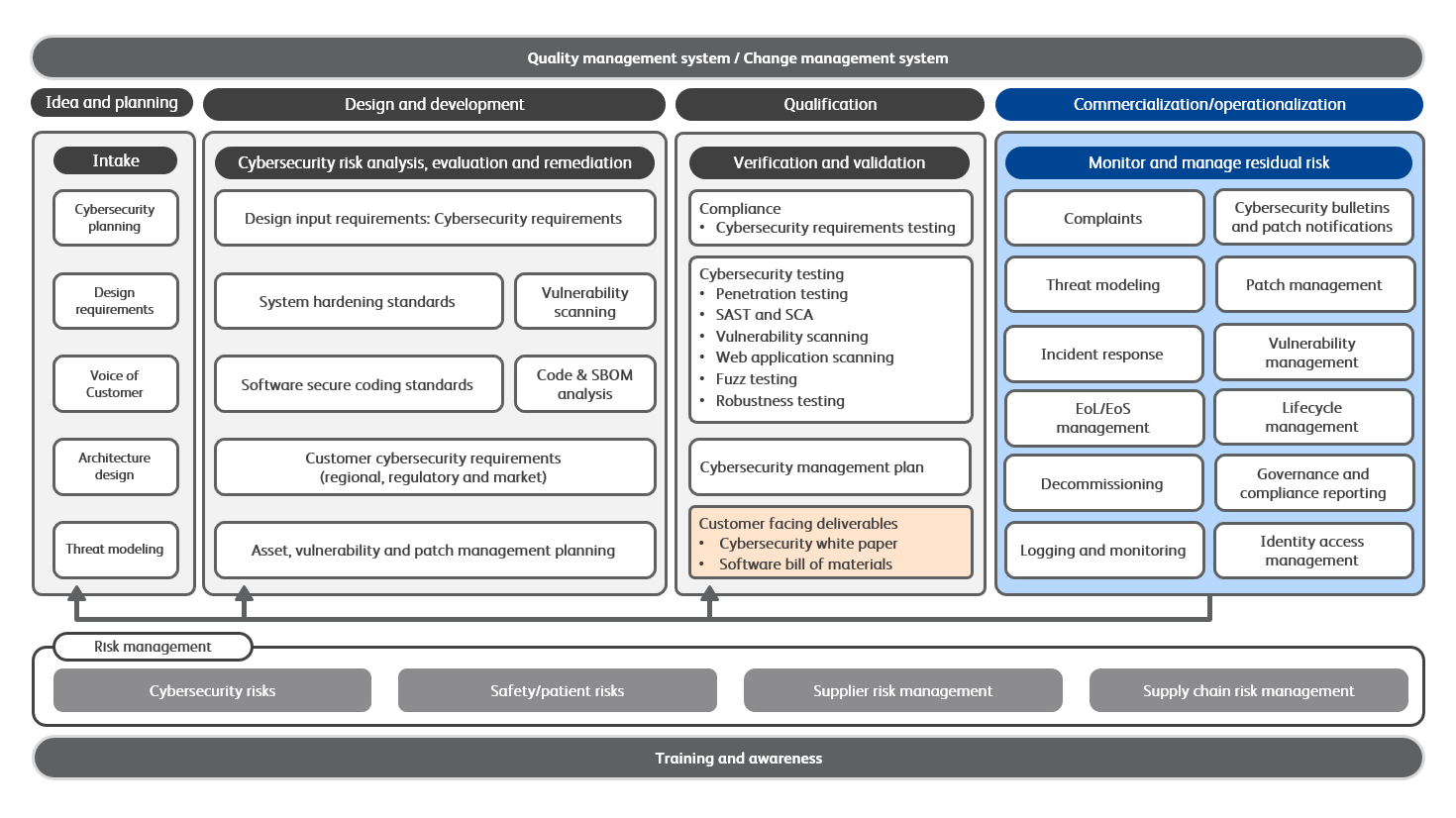
BD Cybersecurity Framework
BD utilizes a framework to incorporate cybersecurity into our processes for product design, manufacturing, customer support and enterprise systems. Our framework has been aligned to various industry work products including the Healthcare & Public Health Sector Coordinating Councils (HSCC) Medical Device and Health IT Joint Security Plan, the National Institute of Standards and Technology (NIST) Cybersecurity Framework, the International Organization for Standardization (ISO) 27001 standards, Underwriters Laboratories (UL) 2900 Standard for Software Cybersecurity for Network-Connectable Products and the International Society of Automation (ISA) 62443.

AdvaMed
The Advanced Medical Technology Association advocates globally for the highest ethical standards and patient access to safe, effective and innovative medical technologies.

AiSP
The Association of Information Security Professionals, based in Singapore, is committed to promoting the development, increase and spread of cybersecurity knowledge.

CCAPAC
The Cybersecurity Coalition for Asia Pacific is dedicated to improving the policy landscape for cybersecurity in Asia.

CVE® Program
BD is authorized as a Common Vulnerability and Exposures (CVE) Numbering Authority by the CVE Program.
DSAC
The Domestic Security Alliance Council is a strategic alliance that includes the U.S. Federal Bureau of Investigation (FBI), Department of Homeland Security and private industry networking together.

H-ISAC
For maximum reach, BD shares coordinated vulnerability disclosures with the Health Information Sharing and Analysis Center.

HSCC
BD participates in multiple Healthcare and Public Health Sector Coordinating Council (HSCC) Cybersecurity Working Group Task Groups.

IMDRF
BD participates in the International Medical Device Regulators Forum with the shared goal of harmonizing medical device cybersecurity around the world.

MDIC
The Medical Device Innovation Consortium works with government and industry stakeholders to advance solutions that promote patient access to innovative medical technologies.

MedTech Europe
BD participates in the MedTech Europe Cybersecurity Working Group, which brings cybersecurity experts together to engage with European institutions, including the European Union Agency for Cybersecurity (ENISA).

U.S. FBI InfraGard
BD participates in the U.S. FBI InfraGard, a partnership between the FBI and the private sector for the purpose of protecting U.S. Critical Infrastructure.
Access BD cybersecurity resources
BD recognizes the value independent cybersecurity attestations provide to our customers. Each year a range of third-party audits are performed on BD products and internal cybersecurity controls. To demonstrate our commitment to protecting BD, our customers and patients, BD makes these industry-recognized certifications and attestation reports available through the BD Cybersecurity Trust Center.
ISO/IEC 27001:2022 is an independently audited certification which demonstrates that an organization meets rigorous international standards for managing information security, including establishing, implementing, maintaining and continually improving its Information Security Management System.
The following ISO/IEC certificates are available for download:
- BD Enterprise and BD Australia ISO/IEC 27001:2022 certificate
- BD Germany ISO/IEC 27001:2022 certificate [ENG] / [GER]
- BD Israel ISO/IEC 27001:2022 certificate: [ENG] / [HEB]
BD maintains a SOC2+ program for multiple BD products that collect and process patient health information in accordance with the HIPAA security rule. These annual audits address the Trust Principles for Security and, for our cloud-based products, Availability. These reports are prepared by an independent third party and provide assurance regarding the operational effectiveness of BD internal controls and the security of BD products. Use the form below to request SOC2+ documents.
UL CAP, which stands for Underwriters Laboratories Cybersecurity Assurance Program, is an independently audited certification that demonstrates the cybersecurity of medical device products through a rigorous program of analysis. UL CAP cybersecurity testing is extensive and challenges BD products against known cybersecurity vulnerabilities, malware, malformed input (fuzz testing), structured penetration, static source code analysis, static binary and bytecode analysis, and verification of security controls (access control, user authentication and authorization, remote communication, cryptography and software updates). The following UL CAP certificates are available for download:
BD maintains Product Security White Papers for its software-enabled products. The purpose of these documents is to provide details on how BD security and privacy practices have been applied and what our customers should know about maintaining security throughout the entire product life cycle. Each white paper includes a Manufacturer Disclosure Statement for Medical Device Security (MDS2 attestation). Use the form below to request Product Security White Papers.
With the exception of UL CAP certificates, the following resources are restricted to existing BD customers and can be requested using the form below. Prospective customers that wish to obtain copies of SOC2+ reports or Product Security White Papers can request these from their sales representative following approval of a Confidential Disclosure Agreement (CDA). Select the documents you would like to access and use the icons at the bottom of the page to trigger the download or request. For additional assistance, please contact BD Customer Support.
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| SOC2+ report for BD Pyxis™ Supply Technologies, BD Pyxis™ Medication Technologies, BD Pyxis™ Remote Support System (RSS) and Coordinated Care Engine (CCE) | |||
| BD Knowledge Portal for BD Pyxis™ Supply Technologies | |||
| BD Pyxis™ SupplyStation system | |||
| BD Cato™ | |||
| BD Knowledge Portal for BD Pyxis™ Medication Technologies | |||
| BD Pyxis™ Anesthesia Station ES | |||
| BD Pyxis™ Anesthesia Station | |||
| BD Pyxis™ CIISafe | |||
| BD Pyxis™ Connect | |||
| BD Pyxis™ DuoStation system | |||
| BD Pyxis™ EcoStation system | |||
| BD Pyxis™ Enterprise Server | |||
| BD Pyxis™ Inventory Connect | |||
| BD Pyxis™ IV Prep | |||
| BD Pyxis™ Logistics system | |||
| BD Pyxis™ MedStation | |||
| BD Pyxis™ ParAssist | |||
| BD Pyxis™ PARx system | |||
| BD Pyxis™ PharmoPack System | |||
| BD Pyxis™ Remote Manager Temp Monitor | |||
| BD Pyxis™ Tissue and Implant System |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| SOC2+ report for the BD Alaris™ System and BD Alaris™ Remote Support System (RSS) and Care Coordination Engine (CCE) | |||
| BD Alaris™ 8015 System | |||
| BD Alaris™ CQI Reporter | |||
| BD Alaris™ Gateway Workstation | |||
| BD Alaris™ neXus CC Syringe Pump | |||
| BD Alaris™ neXus Editor | |||
| BD Alaris™ neXus GP Volumetric Pump | |||
| BD Alaris™ neXus PK Syringe Pump | |||
| BD Alaris™ Technical Utility Software | |||
| BD Alaris™ Communication Engine | |||
| BD BodyComm™ Software | |||
| BD BodyGuard™ Infusion Pump | |||
| BD BodyGuard™ Duo Pump | |||
| BD BodyGuard™ Epidural Pump | |||
| BD BodyGuard™ Pain Manager | |||
| BD BodyGuard™ T Syringe Pump |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| SOC2+ report for BD HealthSight™, BD Knowledge Portal™ and BD Arctic Sun™ Analytics | |||
| BD HealthSight™ Benchmarks | |||
| BD HealthSight™ Clinical Advisor | |||
| BD HealthSight™ Data Manager | |||
| BD HealthSight™ Diversion Management | |||
| BD HealthSight™ Infection Advisor | |||
| BD HealthSight™ Inventory Optimization | |||
| BD HealthSight™ Medication Safety Analytics |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| BD Care Coordination Engine (CCE) | |||
| BD Remote Support Solution (RSS) / BD Remote Assist / BD Assurity Linc™ | |||
| BD Regional Protected Server |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| SOC2+ report for BD BACTEC™, BD Phoenix™, BD Viper™, BD MAX™, BD Kiestra™, BD Focal Point™ Slide Profiler, BD Totalys™, BD COR™ and Informatics Remote Support System (RSS) and Care Coordination Engine (CCE) | |||
| BD BACTEC™ FX Instrument | |||
| BD BACTEC™ FX40 Instrument | |||
| BD BACTEC™ MGIT™ 320 Instrument | |||
| BD BACTEC™ MGIT™ 960 Instrument | |||
| BD MAX™ | |||
| BD Phoenix™ AP | |||
| BD Phoenix™ M50 | |||
| BD Veritor™ Plus |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| SOC2+ report for BD BACTEC™, BD Phoenix™, BD Viper™, BD MAX™, BD Kiestra™, BD Focal Point™ Slide Profiler, BD Totalys™, BD COR™ and Informatics Remote Support System (RSS) and Care Coordination Engine (CCE) | |||
| BD COR™ System | |||
| BD DataLink | |||
| BD FocalPoint™ GS imaging system | |||
| BD Totalys™ Multiprocessor | |||
| BD Totalys™ SlidePrep | |||
| BD Viper™ LT System |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| SOC2+ report for BD BACTEC™, BD Phoenix™, BD Viper™, BD MAX™, BD Kiestra™, BD Focal Point™ Slide Profiler, BD Totalys™, BD COR™ and Informatics Remote Support System (RSS) and Care Coordination Engine (CCE) | |||
| BD Kiestra™ InoqulA | |||
| BD Kiestra™ TLA System | |||
| BD Kiestra™ WCA System |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| BD FACSCalibur™ | |||
| BD FACSCanto™ 10-color | |||
| BD FACSCanto™ II Clinical | |||
| BD FACSCount™ System | |||
| BD FACSDuet™ | |||
| BD FACSLink™ | |||
| BD FACSLyric™ (IVD) | |||
| BD FACSPresto™ | |||
| BD FACS™ Sample Prep Assistant (SPA) III | |||
| BD FACSVia™ | |||
| BD FACS™ Lyse Wash Assistant™ (LWA) | |||
| BD FACS™ Workflow Manager |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| BD Intelliport™ Medication Management System |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| BD Site~Rite™ 8 Ultrasound Systems | |||
| BD Sherlock 3CG+™ Tip Confirmation System |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| BD Arctic Sun™ Analytics | |||
| BD Arctic Sun™ 5000 Temperature Management System | |||
| BD Arctic Sun™ 6000 Stat Temperature Management System |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| BD Senssica™ Urine Output System |
| Product | Request SOC2 report | Download UL CAP certificate | Request BD product security white paper |
|---|---|---|---|
| SOC2+ report for the BD Pyxis™ RapidRx platform | |||
| BD Pyxis™ MedBank | |||
| BD Pyxis™ RapidRX | |||
| BD Rowa™ Dose | |||
| BD Rowa™ vMAX™ |
Coordinated Vulnerability Disclosure
BD has established a routine practice of seeking, communicating and addressing cybersecurity issues in a timely fashion. Vulnerability disclosure is an essential component to our approach to transparency by enabling customers to manage risk properly through awareness and guidance.
Process
BD 2023 Product Security Annual Report
Learn how BD strives to improve the security of our products.
BD 2021 Cybersecurity Annual Report
Download this report to learn more about our approach to cybersecurity.
-
 JUNE 13, 2024
JUNE 13, 2024The report highlights healthcare cybersecurity trends, the company’s methodology for securing BD products and its impact on advancing best practices for medical device cybersecurity.
-
 OCTOBER 29, 2023
OCTOBER 29, 2023As disciplines, cybersecurity and information technology (IT) are built on the same pillars: people, process and technology. Like a three-legged-stool, all three must be present and supported in equal measure for the foundation to be solid.
-
 AUGUST 9, 2023
AUGUST 9, 2023Communicating about potential cybersecurity vulnerabilities helps protect patients and builds trust. Learn how BD has led the way with coordinated vulnerability disclosures.
-
 February 13, 2023
February 13, 2023MedTech companies must be proactive about protecting medical devices from cybersecurity threats.
-
 January 25, 2023
January 25, 2023The third annual BD cybersecurity report highlights the company’s ongoing efforts to advance cybersecurity maturity, protect against cyberattacks and empower customers with information about cyber risks and vulnerabilities.
-
 January 25, 2023
January 25, 2023Find out how BD strives to protect against sophisticated cybersecurity threats.
-
 January 25, 2023
January 25, 2023The health care industry continues to face escalating cybersecurity threats. Find out what trends we expect to see in the year ahead.
-
 January 25, 2023
January 25, 2023Learn about some of the proactive measures BD takes to protect cybersecurity.
-
 October 31, 2022
October 31, 2022While some view people as the weakest link in security, BD CISO Rob Suárez sees them as cybersecurity’s biggest ally.
-
 October 26, 2022
October 26, 2022Our enterprise-level Information Security Management System (ISMS) has been certified to a rigorous set of independently audited international standards: ISO/IEC 27001:2022.
-
 June 2, 2022
June 2, 2022Learn how a new self-assessment tool is helping the industry establish benchmarks for medical device cybersecurity maturity.
-
 January 6, 2022
January 6, 2022The second annual BD cybersecurity report details the state of health care cybersecurity, the company's impact on advancing cybersecurity maturity and anticipated trends for 2022.
-
 October 28, 2021
October 28, 2021Learn how BD fosters a strong cybersecurity culture by providing opportunities to hear directly from customers and partners, conducting phishing simulations and providing ongoing, tailored cybersecurity training.
-
 October 13, 2021
October 13, 2021Two industry experts share strategies for reducing ransomware risks, from training staff to recognize threats to boosting cyberattack preparedness.
-
June 2, 2021
BD becomes the first medical technology company authorized as a Common Vulnerability and Exposures (CVE®) Numbering Authority by the CVE Program, further demonstrating company's leadership in health care cybersecurity.
-
 December 14, 2020
December 14, 2020Through the BD 2020 Cybersecurity Report, the company seeks to address cybersecurity challenges specific to health care and offer guidance about cybersecurity practices for working with medical device manufacturers.
-
 November 16, 2020
November 16, 2020The COVID-19 pandemic continues to place unprecedented demands on the healthcare system. Many hospitals have had to reconfigure their facilities, from expanding remote monitoring and telemedicine to adding dedicated COVID-19 units.
-
 July 22, 2020
July 22, 2020With this growth comes broad and complex challenges, which health care providers, medical device manufacturers and industry regulators must address to secure connected health devices and protect patient safety and privacy.
-
 July 01, 2020
July 01, 2020To improve the resilience of healthcare during a pandemic or any other crisis, we need to adopt Zero Trust principles. In other words, we need to assume nothing and verify everything.
-
 May 22, 2020
May 22, 2020Even as healthcare professionals put their own lives at risk to save patients, cybercriminals are diligently honing their craft. They are exploiting the need for faster, less stringent security vetting processes and conducting their own version of A/B testing to determine which phishing and smishing campaigns are most effective in the current environment.
-
 February 20, 2020
February 20, 2020The BD Synapsys informatics solution is among the first life-science diagnostics informatics platforms to meet all UL CAP cybersecurity standards.
