1. Twardowski ZJ et al “Intraabdominal Pressures during Natural Activities in Patients Treated with Continuous Ambulatory Peritoneal Dialysis” Nephron 44:129-135 1986. Cobb WS et al. "The Argument for Lightweight Polypropylene Mesh in Hernia Repair" Surgical Innovation. Vol 12 (1).63-69, 2005.
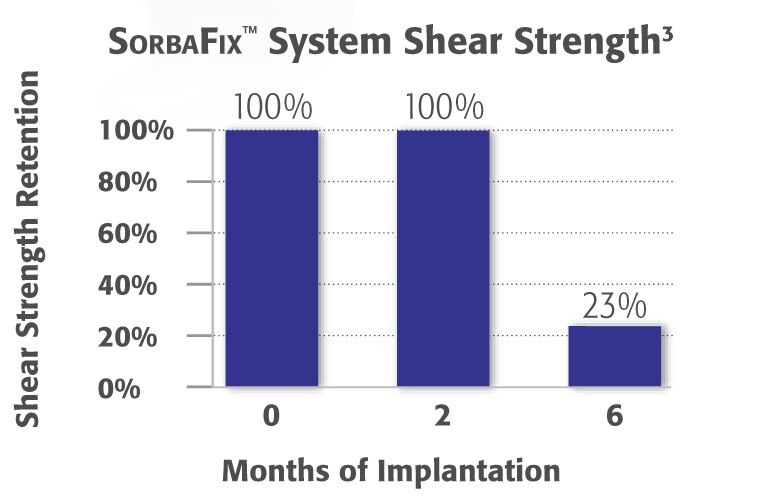
2. Data generated from an animal study using the SORBAFIX™ Absorbable Fixation System and from a cadaver study using the PERMAFIX™ Permanent Fixation System. Data on file. Results may not correlate to performance in humans.
Indications
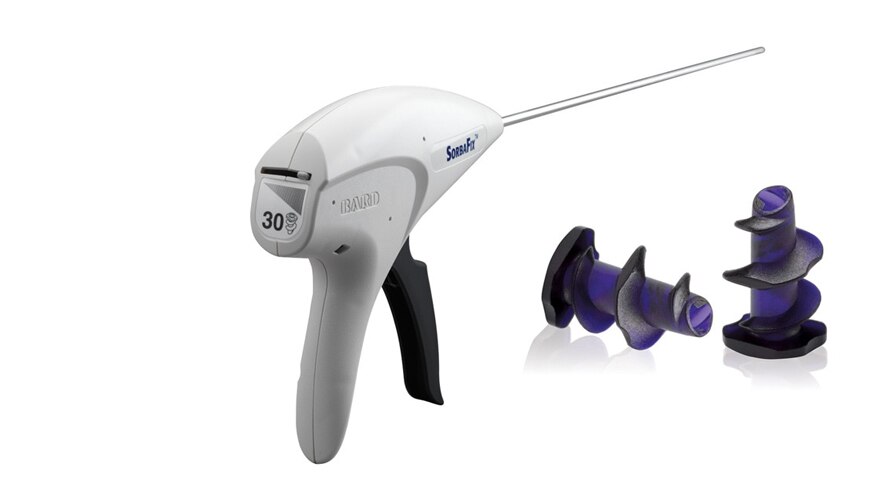
The SorbaFix™ Absorbable Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
Contraindications
Contraindications associated with laparoscopic and open surgical procedures relative to mesh fixation apply including but not limited to:
- Fixation of vascular or neural structures
- Fixation of bone and cartilage
- Situations with insufficient in-growth of tissue into the mesh over time, which could result in inadequate fixation once the fastener is resorbed.
- Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as nerves, vessels, viscera or bone. Use of the SorbaFix™ Absorbable Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener is 6.0 mm, the fastener head is another 0.7 mm (total 6.7 mm).
Warnings
The device may not fixate through prosthetics derived from biologic material such as xenografts and allografts. Prosthetic should be evaluated for compatibility prior to use. To prevent patient injury from the piloting tip, stay clear of vessels, nerves, bowel and viscera when entering the surgical site, manipulating tissue and fixating mesh. After use, the SorbaFix™ Absorbable Fixation System may be a potential biohazard. This device has a piloting tip, which should be considered a sharp even when the device is not actuated. Handle and dispose of in accordance with any local and federal laws regarding medical waste and sharps disposal requirements to prevent sharps injuries.
Adverse Reactions
Adverse reactions and potential complications associated with fixation devices such as the SorbaFix™ Absorbable Fixation System may include, but are not limited to the following: hemorrhage; pain, edema and erythema at wound site; allergic reaction to Poly (D, L)-lactide; septicemia/infection; hernia recurrence/wound dehiscence.
Please consult package insert for more detailed safety information and instructions for use.