-

BD PhaSeal™ Optima Protector P28-O
SKU/REF 515063
-

BD PhaSeal™ Optima Infusion Clamp M25-O
SKU/REF 515085
-

BD PhaSeal™ Optima Infusion Adapter C100-O Multipack
SKU/REF 515079
-

BD PhaSeal™ Optima Infusion Adapter C100-O
SKU/REF 515078
-

BD PhaSeal™ Optima Infusion Connector C35-O
SKU/REF 515070
-

BD PhaSeal™ Optima Protector P20-O Multipack
SKU/REF 515065
-

BD PhaSeal™ Optima Protector P20-O
SKU/REF 515064
-

BD PhaSeal™ Optima Protector P13-O
SKU/REF 515060
-

BD PhaSeal™ Optima Injector N40-O Multipack
SKU/REF 515057
-

BD PhaSeal™ Optima Injector N40-O
SKU/REF 515056
-

BD PhaSeal™ Optima Injector N35-O Multipack
SKU/REF 515053
-

BD PhaSeal™ Optima Injector N35-O
SKU/REF 515052
-

BD® Closed System Drug Transfer Device Assembly Fixture M12-O
SKU/REF 515082
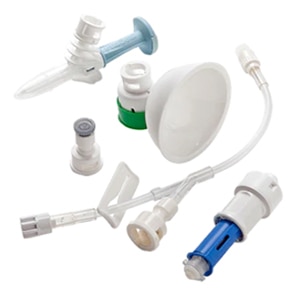
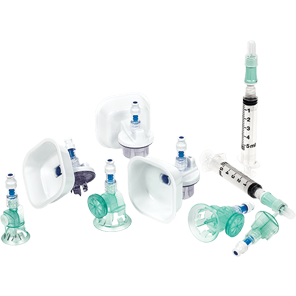
BD PhaSeal™ Optima system
The next-generation Closed-System Drug-Transfer Device (CSTD) from BD that advances hazardous drug protection


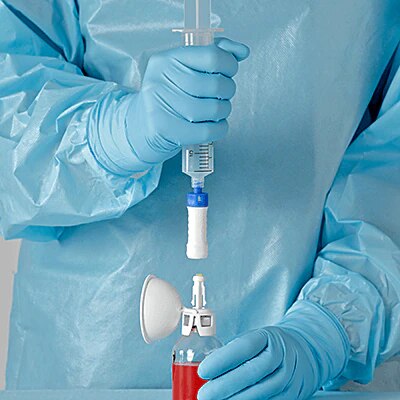
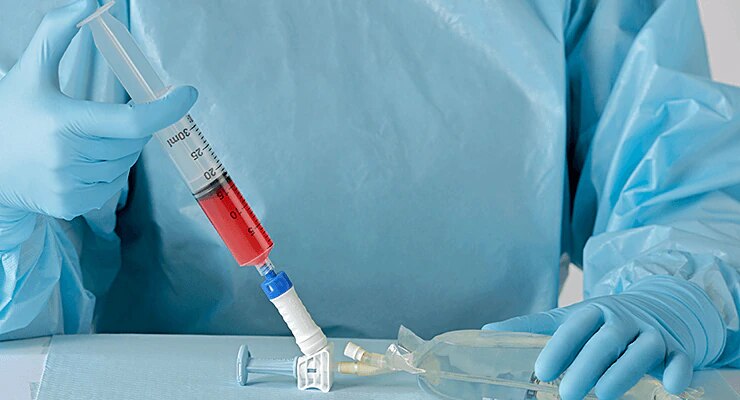
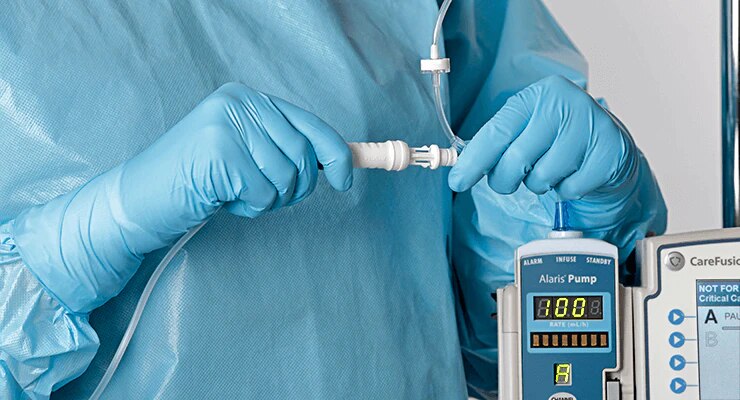

- Overview
- Product & Accessories
- EIFU & Resources
Airtight
Every component is designed with no inlets or air exchange for completely airtight hazardous drug transfers.
Demonstrated
The BD PhaSeal™ Optima system prevents microbial ingress for up to 168 hours and 10 penetrations*†
Efficient
The BD PhaSeal™ Optima system minimizes residual drug loss in vials compared to other similar system.
User-validated
Feedback from longstanding CSTD users and traditional technique users guided us to optimize and innovate the design.
Safety
- Injector designed to prevent accidental needlesticks
- Self-sealing membranes for leakproof connections
Performance
- Proprietary Protector design to maximize drug extraction
- Compatible with ISO-compliant Luer lock syringes and IV sets
Ergonomics
- Power grip and straight motion to connect and disconnect, aligned with NIOSH recommendations to avoid pinch grip3
- Optimized design for clinician comfort
Ease of use
- Intuitive one-step straight-push connection to aid workflow
- Can connect without alignment to help minimize learning curve
BD PhaSeal™ Optima Video for Pharmacy
BD PhaSeal™ Optima for Nursing
-

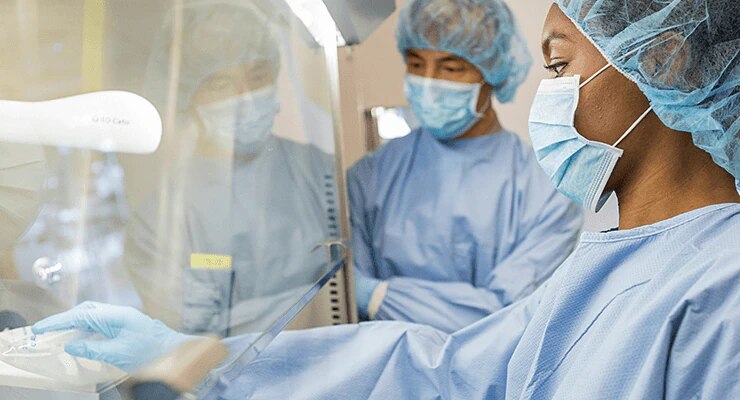
Protect clinicians and patients from exposure to hazardous drugs with a closed system
-

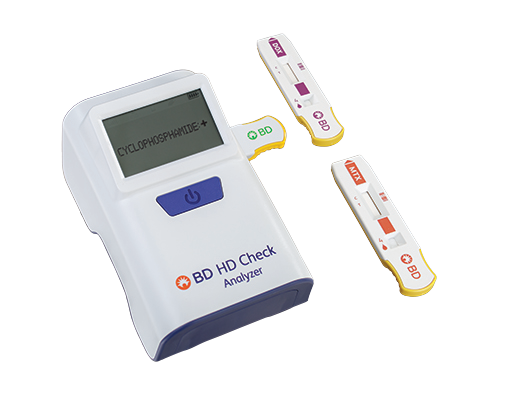
Get results in less than 10 minutes with the first and only rapid hazardous drug* detection system
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Notes
‡2015
*Within an ISO Class V environment following aseptic technique.
†The ability to prevent microbial ingress for up to 168 hours should not be interpreted as modifying, extending or superseding a manufacturer's labeling recommendations for the storage and expiration dating of the drug vial. Refer to drug manufacturer’s recommendations and USP compounding guidelines for shelf life and sterility information.
References
- National Institute for Occupational Safety and Health (NIOSH). A vapor containment performance protocol for closed system transfer devices used during pharmacy compounding and administration of hazardous drugs. https://www.cdc.gov/niosh/docket/review/docket288/pdfs/a-vapor-containment-performance-protocol-for-closed-system-transfer-devices.pdf. Accessed May 26, 2020.
- BD-8440 BD PhaSeal Optima Vapor Containment White Paper.
- Easy Ergonomics: A Guide to Selecting Non-Powered Hand Tools. Centers for Disease Control and Prevention website. https://www.cdc.gov/niosh/docs/2004-164/pdfs/2004-164.pdf. Published August 18, 2004. Accessed April 22, 2020.
BD-20816