관련 제품을 사용할 수 없음
true
Support
Sales
1.844.8.BD.LIFE (1.844.823.5433)
Thank you for contacting our sales team!
A sales representive will get in touch with you shortly.
Ordering
1.800.556.6756
BDISurgery@bd.com
Onsite Visiting
100 Crossings BoulevardWarwick, Rhode Island 02886United States
Customer Service
1.844.8.BD.LIFE (1.844.823.5433)
Monday to Friday 8:15 AM to 6:00 PM EST
1.800.531.4124
Custserv.davol@bd.com
Shipping a Product Back
100 Crossings Boulevard, Warwick, Rhode Island 02886, United States


- 개요
- 제품 및 부속품
- EIFU 및 리소스
- 자주 묻는 질문(FAQ)
간편합니다.
안전합니다.
효과적입니다.
Arista™ AH는 정제된 식물의 전분에서 유래한 100% 식물성 흡수성 수술용 지혈 분말입니다. Arista™ AH의 힘은 특허 받은 혈액 응고 기술인 MPH™(미소공성 다당류 헤모스피어)에 있습니다.
Arista™ AH는 압박, 결찰 또는 다른 통상적인 절차를 통해 모세혈관, 정맥 또는 세동맥 출혈을 효과적으로 제어할 수 없거나 제어가 불가능한 경우에 지혈을 돕는 보조 지혈 기구로 외과적 수술(안과 수술 제외)에서 사용됩니다.
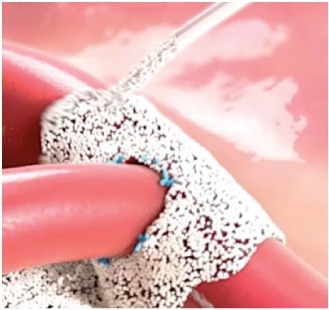
간편합니다
- 혼합 및 냉장 보관 불필요
- 바로 사용 가능 및 유효 기간 5년
- 캡을 열고 분말을 출혈 부위에 바로 적용2
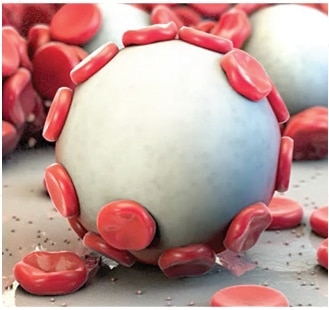
안전합니다
- 트롬빈 불포함, 생체 적합적이며 비발열성
- 일반적으로 아밀라아제로 24~48시간* 이내 흡수 및 제거
*신생아부터 생후 10개월까지 아밀라아제 활성도가 감소되었다는 보고가 있었기 때문에 이 모집단에서 Arista™ AH의 흡수율은 48시간 이상일 수 있습니다.
독점 MPH™ 기술: 지혈을 위한 독특한 접근법
Arista™ AH의 힘은 독점 MPH™(미소공성 다당류 헤모스피어) 기술에 있습니다. 제어된 기공 크기의 미소공성 입자로 구성된 구체는 분자체로 작용하도록 설계되었습니다. 강력한 삼투 작용은 접촉한 혈액을 건조하고 젤화하여 자연적인 응고 과정을 가속화합니다.

효과적입니다
- 환자의 응고 상태에 관계 없이 접촉 시 응고 과정 시작3
- 몇 분 내로 완전한 지혈 가능1
- 거친 표면과 손이 닿기 힘든 부분에 넓은 적용 범위 제공
1 Arista™ AH PMA P050038 Clinical Study.
2 See full Instructions for Use for detailed application instructions.
3 Arista™ AH Instructions for Use.
INDICATIONS
Arista™ AH is indicated in surgical procedures (except ophthalmic) as an adjunctive hemostatic device to assist when control of capillary, venous, and arteriolar bleeding by pressure, ligature, and other conventional procedures are ineffective or impractical.
CONTRAINDICATIONS
Do not inject or place Arista™ AH into blood vessels as potential for embolization and death may exist.
WARNINGS
Arista™ AH is not intended as a substitute for meticulous surgical technique and the proper application of ligatures or other conventional procedures for hemostasis.
Once hemostasis is achieved, excess Arista™ AH should be removed from the site of application by irrigation and aspiration particularly when used in and around foramina of bone, areas of bony confine, the spinal cord, and/or the optic nerve and chiasm. Arista™ AH swells to its maximum volume immediately upon contact with blood or other fluids. Dry, white Arista™ AH should be removed. The possibility of the product interfering with normal function and/or causing compression necrosis of surrounding tissues due to swelling is reduced by removal of excess dry material.
Safety and effectiveness of Arista™ AH have not been clinically evaluated in children and pregnant women. Because there have been reports of decreased amylase activity in newborns up to 10 months, absorption rates of Arista™ AH in this population may be longer than 48 hours.
Arista™ AH should be used with caution in the presence of infection or in contaminated areas of the body. If signs of infection or abscess develop where Arista™ AH has been applied, re-operation may be necessary in order to allow drainage.
Safety and effectiveness in neurosurgical and ophthalmic procedures has not been established.
Arista™ AH should not be used for controlling post-partum bleeding or menorrhagia.
PRECAUTIONS
When Arista™ AH is used in conjunction with autologous blood salvage circuits, carefully follow instructions in the Administration section of the IFU regarding proper filtration and cell washing.
Arista™ AH is intended to be used in a dry state. Contact with saline or antibiotic solutions prior to achieving hemostasis will result in loss of hemostatic potential.
Arista™ AH is not recommended for the primary treatment of coagulation disorders.
No testing has been performed on the use of Arista™ AH on bone surfaces to which prosthetic materials are to be attached with adhesives and is therefore not recommended.
Arista™ AH is supplied as a sterile product and cannot be resterilized. Unused, open containers of Arista™ AH should be discarded.
Do not apply more than 50g of Arista™ AH in diabetic patients as it has been calculated that amounts in excess of 50g could affect the glucose load.
In urological procedures, Arista™ AH should not be left in the renal pelvis or ureters to eliminate the potential foci for calculus formation.
ADVERSE REACTIONS
None of the adverse events that occurred in a randomized prospective, concurrently controlled clinical trial were judged by the Data Safety Monitoring Board to be related to the use of Arista™ AH. The most common recorded adverse events were pain related to surgery, anemia, nausea, lab values out of normal range, arrhythmia, constipation, respiratory dysfunction and hypotension – all reported in greater than 5% of the Arista™ AH treated patients. The details of this clinical trial’s adverse events can be reviewed in the IFU supplied with the product and are also available at www.bd.com.
BD-126650
true
The Basics of Arista™ AH Absorbable Hemostatic Particles
- Topical hemostats include agents that act as a mechanical barrier to bleeding and provide a physical matrix for clotting, biologically active agents that catalyze coagulation, and combination therapies. 2
- Hemostats can enhance clot formation and wound healing, and can be useful for controlling or preventing troublesome bleeding during surgical interventions where conventional methods of hemostasis are inadequate.2
- AH is an absorbable powdered hemostatic agent intended for application to surgical wound sites to control bleeding. It is a fine, dry, sterilized white powder that is biocompatible, non-pyrogenic, and typically absorbed within 24-48 hours."
- Arista™ AH is a 100% plant based absorbable surgical hemostatic powder derived from purified plant starch. The power of Arista™ AH lies in its Microporous Polysaccharide Hemospheres, a patented blood clotting technology. Consisting of microporous particles with a controlled pore size, the spheres are designed to act as a molecular sieve. The powerful osmotic action dehydrates and gels the blood on contact to accelerate the natural clotting process."
How Arista™ AH Works & Is Used
- The acronym “RAPID” can be used to remember the application steps:
- R (Remove): Remove all access blood
- A (Apply): Apply AristaTM AH liberally to the bleeding site
- P (Pressure): Administer wound-appropriate pressure until hemostasis is achieved
- I (Irrigate): Irrigate and remove excess Arista™ AH from the site
- D (Done): Hemostasis Hemostasis achieved— quickly, safely and effectively
- With the FlexiTip™ spray applicator, applying Arista™ AH accurately and directly is fast and simple. The delivery system features a lightweight, plastic device with a long, flexible tube. The FlexiTip™ and FlexiTip™ XL extended reach applicator tips provide the precise and accurate delivery of Arista™ AH hemostatic powder from a simple, single-use device.1
- Arista Application: see instructions for use 1
- Directions for Use:
- Blot, wipe, or suction the bleeding tissue. It is important to remove excess blood so Arista™ AH may be applied immediately and directly to the site of active bleeding.
- Position the applicator tip as close to the source of bleeding as possible. Immediately apply a liberal amount of Arista™ AH at the site of bleeding within the wound, to completely cover the wound. Deep wounds may require equally deep application of Arista™ AH. To minimize occlusion of the tip, pressure should be applied to deliver Arista™ AH as the applicator enters the wound
- Quickly apply wound-appropriate, direct pressure over the treated site. Use of a non-adhering substrate to apply pressure may prevent adhesion of the formed clot to the surgical glove or other instrumentation. Amount and duration of pressure is wound dependent. For oozing, pressure may not be necessary. For more profusely bleeding wounds, pressure should be maintained longer.
- If bleeding or oozing continues, remove excess Arista™ AH and reapply.
- If any material other than the clot-bound Arista™ AH (i.e. surgical dressing) adheres to the wound site, irrigate the material with saline and carefully remove it from the treated site.
- Immediately upon contact with blood or fluid, Arista™ AH will swell to approximately 5 times its original volume. Once hemostasis is achieved, excess Arista™ AH should be carefully removed by irrigation and aspiration. Avoid irrigation of direct suction of the formed blood clot.1
- Administration: A liberal amount of Arista™ AH should be applied to the bleeding site (see DIRECTIONS FOR USE) followed by pressure until hemostasis is achieved. After hemostasis is achieved, Arista™ AH should be removed by irrigation and/or aspiration.1
Examples of surgeries that Arista™ AH can be used in include:
- Cardiothoracic and cardiovascular
- Vascular
- Gynecological
- Urology
- Orthopedic surgery
- General surgery
- Plastic surgery
- Ear
- nose
- throat (ENT) surgery
Advantages of Arista™ AH
- A wide variety of topical hemostats are approved as adjunctive therapies in the maintenance of hemostasis during surgical procedures in which conventional methods are insufficient or not practical.2
- Potential cost benefits in terms of preventing the further utilization of hospital resources to manage surgical bleeding. In addition to other patient blood management strategies, the intraoperative use of topical hemostats may reduce the need for transfusion of blood products (e.g., packed red blood cells, platelets, fresh frozen plasma) and reduce the duration of postoperative care (length of hospital stay) each of which is associated with substantial costs.2
Simple to Use
- Ready on demand
- Simply pop the cap and apply the powder directly to the bleeding site
- No mixing and no refrigeration
- Five-year shelf life4
Safe and Thrombin-Free
- Synthesized from a purified plant starch
- Thrombin-free, biocompatible and nonpyrogenic
- Typically absorbed and cleared within 24–48 hours by amylases
- Arista™ AH is the only currently available hemostat that is approved for cell salvage compatibility1,6,7
Effective Hemostat
- The clotting process begins on contact, regardless of patient’s coagulation status
- Complete hemostasis can be achieved in minutes5
- Provides broad area coverage
Arista™ AH Results and Case Studies
PMA clinical trial (General, Orthopedic, Cardiac)
Cardiac & Vascular:
- Bruckner et al. (2014) Microporous polysaccharide hemosphere absorbable hemostat use in cardiothoracic surgical procedures
- Reynbakh et al. (2018) Use of MPH hemostatic powder for electrophysiology device implantation reduces postoperative rates of pocket hematoma and infection
- Bruckner and Loebe (2012a) Microporous polysaccharide hemosphere absorbable hemostat (AristaAH) use in re-operative cardiac surgical procedures.
- Bruckner and Loebe (2012b) The use of an absorbable microporous polysaccharide hemosphere haemostat (AristaAH) in ventricular assist device implant and cardiac transplantation procedures.
- Benlier et al. (2007) Microvascular anastomosis with minimal suture and arista: An experimental study
ENT:
- Antisdel et al. (2011) Microporous polysaccharide hemospheres do not increase synechiae after sinus surgery: Randomized controlled study
- Antisdel et al. (2009a) Effect of hemostatic Agent MPH on bleeding after endoscopic sinus surgery: A prospective randomized controlled study
- Antisdel et al. (2016) Product comparison model in otolaryngology: Equivalency analysis of absorbable hemostatic agents after endoscopic sinus surgery
- Phillips (2013) Safety and efficacy of Arista powder in 514 nasal septal and sinus surgery patients
- Sindwani (2009) Use of novel hemostatic powder MPH for endoscopic sinus surgery: Initial impressions
- Antisdel et al. (2008) Hemostatic agent Microporous Polysaccharide Hemospheres (MPH) does not affect healing or intact sinus mucosa
General:
- Egeli et al. (2012) Microporous polysaccharide hemospheres and seroma formation after mastectomy and axillary dissection in rats
- Humphreys et al. (2008a) Microporous polysaccharide hemospheres for management of laparoscopic trocar injury to the spleen
- Humphreys et al. (2008b) Renal injury and the application of polysaccharide hemospheres: A laparoscopic experimental model
- Hoffmann et al. (2009) Choice of Hemostatic Agent Influences Adhesion Formation in a Rat Cecal Adhesion Mode
- Ereth et al. (2009) Microporous polysaccharide hemospheres do not enhance abdominal infection in a rat model compared with gelatin matrix
Urology
- Nunez-Nateras et al. (2013) Athermal nerve sparing robot-assisted radical prostatectomy: Initial experience with microporous polysaccharide hemospheres as a topical hemostatic agent
- Gilbert et al. (2016) Evaluation of Absorbable Hemostatic Powder for Prevention of Lymphoceles Following Robotic Prostatectomy With Lymphadenectomy
- Murat et al. (2006) Evaluation of microporous polysaccharide hemospheres for parenchymal hemostasis during laparoscopic partial nephrectomy in the porcine model
- Arista™ AH IFU
- Gabay, M. & Boucher, B.A. An essential primer for understanding the role of topical hemostats, surgical sealants, and adhesives for maintaining hemostasis. Pharmacotherapy 33, 935-955 (2013).
- Safety and effectiveness of ARISTA™ AH have not been clinically evaluated in children and pregnant women. Because there have been reports of decreased amylase activity in newborns up to 10 months, absorption rates of ARISTA™ AH in this population may be longer than 48 hours
- Data on file
- ARISTA™ AH PMA Clinical Study P050038
- BD Data on file. Preclinical data may not correlate to clinical performance in humans.
- When Arista™ AH is used in conjunction with autologous blood salvage circuits, a 40 μ cardiotomy reservoir, cell washing, and 40 μ transfusion filter must be used.
BD-53859
true
true