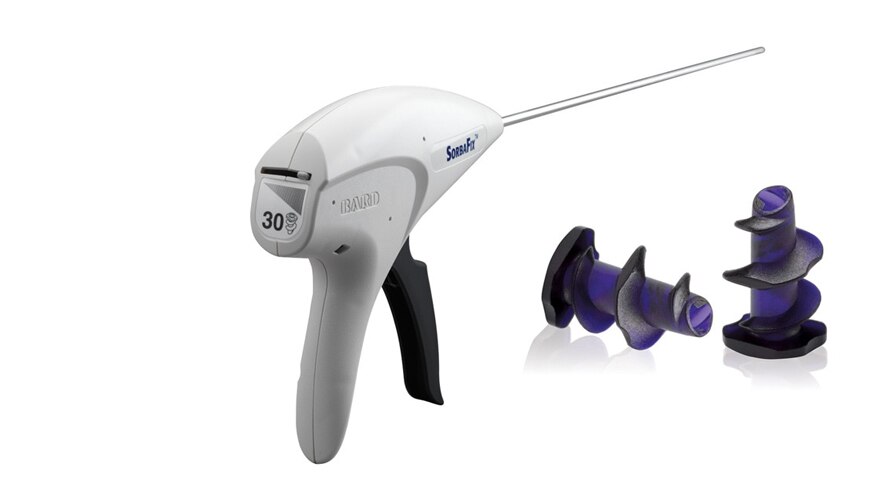
The unique fastener design of the SorbaFix™ Absorbable Fixation system means no sharp points are left behind in the patient. Preclinical data shows that a hollow core allows tissue in-growth through the fastener.2 The 5 mm depth of tissue purchase and consistent thread diameter from head to tip assure maximum tissue engagement during open and laparoscopic procedures. TheSorbaFix™ Absorbable Fixation System has a 5 mm, low-profile delivery system that offers smooth, efficient deployment. Keeping track of the pre-loaded 15- or 30-fastener configurations is now easy with a new fastener gauge.

- Übersicht
- Products & Accessories
- EIFU & weitere Informationen
Smart Design. Not Sharp Design.
Stabil:
- Die Reparaturstärke ist etwa um das 7-Fache größer als der maximale intraabdominelle Druck (TAP).1,2
- Ein Hohlkern mit Gewinde ermöglicht das Einwachsen von Gewebe in das Innere der Fixationselemente.2
Stabil:
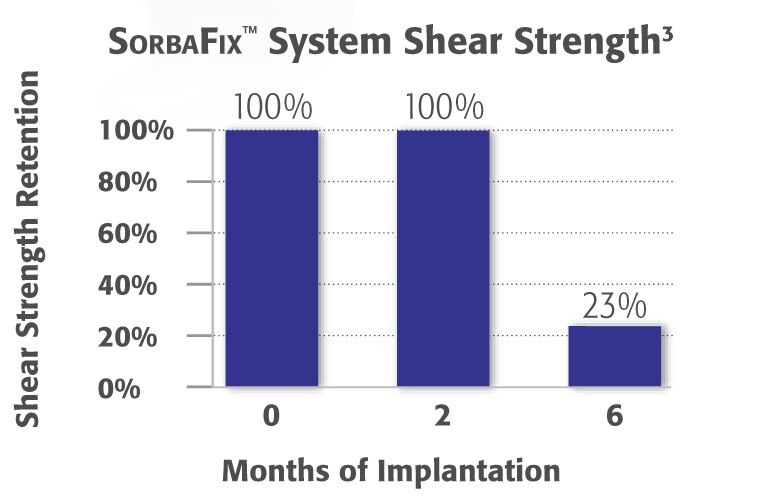
Die Beibehaltung der Scherfestigkeit veranschaulicht den Abbau der SorbaFix™ Fixationselemente im Laufe der Zeit. Die SorbaFix™ Fixationselemente behalten ihre Festigkeit innerhalb der ersten zwei Monate bei und bieten so eine sichere Fixation zu Beginn der Heilungsphase.2
Präklinische Daten. Die Ergebnisse entsprechen nicht zwingend der Leistung im Menschen.
Einheitlich:
- Der einheitliche Gewindedurchmesser vom Kopf bis zu Spitze sowie die Länge der Fixationselemente wurden entwickelt, um eine maximale Gewebeaufnahme zu erreichen
- Der Obturator mit Führungsspitze führt die Fixationselemente präzise durch das Netz und das Gewebe
Zuverlässig:
- Atraumatische Fixationselemente mit stumpfer Spitze, glattem Kopf und ohne freiliegende Spitzen
- Das innovative mechanische Design ermöglicht ein langlebiges Einführsystem.
- Die Resorption der Poly(D,L)-Lactid(PDLLA)-Fixationselemente ist nahezu 12 Monate nach der Implantation abgeschlossen und es bleibt weniger Fremdmaterial zurück.2
Twardowski ZJ et al “Intraabdominal Pressures during Natural Activities in Patients Treated with Continuous Ambulatory Peritoneal Dialysis” Nephron 44:129-135 1986. Cobb WS et al. "The Argument for Lightweight Polypropylene Mesh in Hernia Repair" Surgical Innovation. Vol 12 (1).63-69, 2005.
Data generated from an animal study using the SORBAFIX™ Absorbable Fixation System and from a cadaver study using the PERMAFIX™ Permanent Fixation System. Data on file. Results may not correlate to performance in humans.
Indications
The SorbaFix™ Absorbable Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during laparoscopic surgical procedures, such as hernia repair.
Contraindications
This device is not intended for use except as indicated.
Do not use this device where hemostasis cannot be verified visually after application.
Contraindications associated with laparoscopic surgical procedures relative to mesh fixation apply, including but not limited to: • Fixation of vascular or neural structures • Fixation of bone and cartilage • Situations with insufficient in-growth of tissue into the mesh over time, which could result in inadequate fixation once the fastener is resorbed.
Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as nerves, vessels, viscera, or bone. Use of the SorbaFix™ Absorbable Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener is 6.0 mm, the fastener head is another 0.7 mm (total 6.7 mm).
Warnings
The SorbaFix™ Absorbable Fixation System is intended for Single Use Only – DO NOT RESTERILIZE. Reuse, reprocessing, re-sterilization, or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, re-sterilization, or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient or end user. This product is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
Do not use beyond the expiration date on the package.
Do not use if the center of the temperature indicator is black.
As with any implant material the presence of bacterial contamination may enhance bacterial infectivity. Accepted surgical practices must be followed with respect to drainage and closure of infected or contaminated wounds.
Users should be familiar with surgical procedures and techniques involving synthetic absorbable materials before employing SorbaFix™ Absorbable Fixation System fasteners for wound closure, as the risk of wound dehiscence may vary with the site of application and the material used.
The device may not fixate through prosthetics derived from biologic material such as xenografts and allografts. Prosthetics should be evaluated for compatibility prior to use. To prevent patient injury from the piloting tip, stay clear of vessels, nerves, bowel, and viscera when entering the surgical site, manipulating tissue, and fixating mesh.
To prevent patient injury from the piloting tip, stay clear of vessels, nerves, bowel, and viscera when entering the surgical site, manipulating tissue, and fixating mesh. After use, the SorbaFix™ Absorbable Fixation System may be a potential biohazard. This device has a piloting tip, which should be considered a sharp even when the device is not actuated. Handle and dispose of in accordance with any local and federal laws regarding medical waste and sharps disposal requirements to prevent sharps injuries.
Precautions
Please read all instructions before using the SorbaFix™ Absorbable Fixation System.
Only persons having adequate medical training and familiarity with surgical techniques should perform surgical procedures. Consult the medical literature relative to technique, complications, and hazards prior to any surgical procedure.
The SorbaFix™ Absorbable Fixation System can be used with most 5 mm trocars. Ensure compatibility by inserting the device into the trocar prior to introduction into the patient. The SorbaFix™ Absorbable Fixation System should enter and exit the trocar easily without excessive force. The use of too much force could damage the instrument.
Counter pressure should be applied on the target area. Avoid placing hand/finger directly over the area where the fastener is being deployed to prevent injury.
Insertion of fasteners into some collagenous structures such as ligaments and tendons is possible but is NOT possible directly into bone or cartilage. This may damage the device.
Avoid excessive trigger force as this may damage the device.
If the device locks, remove the device from the patient and lightly tap the trigger forward toward the tip to release.
If the device locks and cannot be separated from a fastener that has been deployed into tissue, you may rotate the device counterclockwise to free the device. The locked device should then be discarded, and a new device should be used.
If the fastener does not deploy properly, remove the device from the patient and test the device in air to ensure proper fastener deployment. Once proper fastener deployment is confirmed, the device may be reinserted into the patient.
The safety and effectiveness of SorbaFix™ Absorbable Fixation System have not been evaluated or established in pregnant or breast feeding women.
This device contains the following substance(s) defined as CMR 1B in a concentration above 0.1% weight by weight: Cobalt; CAS No. 7440-48-4; EC No. 231-158-0. Current scientific evidence supports that medical devices manufactured from stainless-steel alloys containing cobalt do not cause an increased risk of cancer or adverse reproductive effects. For more information, please consult the ECHA website:
Homepage - ECHA .
Adverse Reaction
Adverse reactions and potential complications associated with fixation devices such as the SorbaFix™ Absorbable Fixation System may include, but are not limited to the following: hemorrhage; pain, edema, and erythema at wound site; allergic reaction to Poly (D, L)-lactide; septicemia/infection; hernia recurrence/wound dehiscence. Please consult package insert for more detailed safety information and instructions for use.
Hier finden Sie eine hilfreiche Sammlung weiterer Informationsmaterialien zu Ihrer Verfügung.
BD bietet Schulungsmaterialien an, die Sie bei der Verbesserung Ihrer klinischen Praxis unterstützen und Teil unseres Ziels sind, Fortschritt für die Welt der Gesundheit™ zu bringen.
BD ist Gastgeber und Teilnehmer von Veranstaltungen, die Forschritt für die Welt der Gesundheit™ bringen.
BD unterstützt klinische Spitzenleistungen durch die Bereitstellung von Ressourcen zu bewährten Verfahren, klinischen Innovationen und Branchentrends im Gesundheitswesen.
Twardowski ZJ et al “Intraabdominal Pressures during Natural Activities in Patients Treated with Continuous Ambulatory Peritoneal Dialysis” Nephron 44:129-135 1986. Cobb WS et al. "The Argument for Lightweight Polypropylene Mesh in Hernia Repair" Surgical Innovation. Vol 12 (1).63-69, 2005.
Data generated from an animal study using the SORBAFIX™ Absorbable Fixation System and from a cadaver study using the PERMAFIX™ Permanent Fixation System. Data on file. Results may not correlate to performance in humans.
Indications
The SorbaFix™ Absorbable Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during laparoscopic surgical procedures, such as hernia repair.
Contraindications
This device is not intended for use except as indicated.
Do not use this device where hemostasis cannot be verified visually after application.
Contraindications associated with laparoscopic surgical procedures relative to mesh fixation apply, including but not limited to: • Fixation of vascular or neural structures • Fixation of bone and cartilage • Situations with insufficient in-growth of tissue into the mesh over time, which could result in inadequate fixation once the fastener is resorbed.
Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as nerves, vessels, viscera, or bone. Use of the SorbaFix™ Absorbable Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener is 6.0 mm, the fastener head is another 0.7 mm (total 6.7 mm).
Warnings
The SorbaFix™ Absorbable Fixation System is intended for Single Use Only – DO NOT RESTERILIZE. Reuse, reprocessing, re-sterilization, or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, re-sterilization, or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient or end user. This product is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
Do not use beyond the expiration date on the package.
Do not use if the center of the temperature indicator is black.
As with any implant material the presence of bacterial contamination may enhance bacterial infectivity. Accepted surgical practices must be followed with respect to drainage and closure of infected or contaminated wounds.
Users should be familiar with surgical procedures and techniques involving synthetic absorbable materials before employing SorbaFix™ Absorbable Fixation System fasteners for wound closure, as the risk of wound dehiscence may vary with the site of application and the material used.
The device may not fixate through prosthetics derived from biologic material such as xenografts and allografts. Prosthetics should be evaluated for compatibility prior to use. To prevent patient injury from the piloting tip, stay clear of vessels, nerves, bowel, and viscera when entering the surgical site, manipulating tissue, and fixating mesh.
To prevent patient injury from the piloting tip, stay clear of vessels, nerves, bowel, and viscera when entering the surgical site, manipulating tissue, and fixating mesh. After use, the SorbaFix™ Absorbable Fixation System may be a potential biohazard. This device has a piloting tip, which should be considered a sharp even when the device is not actuated. Handle and dispose of in accordance with any local and federal laws regarding medical waste and sharps disposal requirements to prevent sharps injuries.
Precautions
Please read all instructions before using the SorbaFix™ Absorbable Fixation System.
Only persons having adequate medical training and familiarity with surgical techniques should perform surgical procedures. Consult the medical literature relative to technique, complications, and hazards prior to any surgical procedure.
The SorbaFix™ Absorbable Fixation System can be used with most 5 mm trocars. Ensure compatibility by inserting the device into the trocar prior to introduction into the patient. The SorbaFix™ Absorbable Fixation System should enter and exit the trocar easily without excessive force. The use of too much force could damage the instrument.
Counter pressure should be applied on the target area. Avoid placing hand/finger directly over the area where the fastener is being deployed to prevent injury.
Insertion of fasteners into some collagenous structures such as ligaments and tendons is possible but is NOT possible directly into bone or cartilage. This may damage the device.
Avoid excessive trigger force as this may damage the device.
If the device locks, remove the device from the patient and lightly tap the trigger forward toward the tip to release.
If the device locks and cannot be separated from a fastener that has been deployed into tissue, you may rotate the device counterclockwise to free the device. The locked device should then be discarded, and a new device should be used.
If the fastener does not deploy properly, remove the device from the patient and test the device in air to ensure proper fastener deployment. Once proper fastener deployment is confirmed, the device may be reinserted into the patient.
The safety and effectiveness of SorbaFix™ Absorbable Fixation System have not been evaluated or established in pregnant or breast feeding women.
This device contains the following substance(s) defined as CMR 1B in a concentration above 0.1% weight by weight: Cobalt; CAS No. 7440-48-4; EC No. 231-158-0. Current scientific evidence supports that medical devices manufactured from stainless-steel alloys containing cobalt do not cause an increased risk of cancer or adverse reproductive effects. For more information, please consult the ECHA website:
Homepage - ECHA .
Adverse Reaction
Adverse reactions and potential complications associated with fixation devices such as the SorbaFix™ Absorbable Fixation System may include, but are not limited to the following: hemorrhage; pain, edema, and erythema at wound site; allergic reaction to Poly (D, L)-lactide; septicemia/infection; hernia recurrence/wound dehiscence. Please consult package insert for more detailed safety information and instructions for use.
BD-114860