-

BD Microtainer® Contact-Activated Lancet
SKU/REF 366592
-

BD Microtainer® Contact-Activated Lancet
SKU/REF 366593
-

BD Microtainer® Contact-Activated Lancet
SKU/REF 366594
true
Support
Sales
1.844.8.BD.LIFE (1.844.823.5433)
Thank you for contacting our sales team!
A sales representive will get in touch with you shortly.
Customer Service
1.800.638.8663
technical_services@bd.com


- Overview
- Products & Accessories
- EIFU & Resources
false
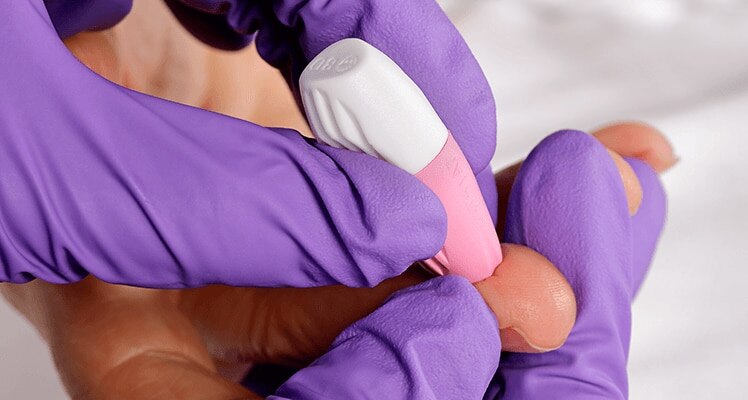
Ergonomic design
The lancet enhances user comfort with an ergonomic design for a more comfortable grip.

Easier sampling
It activates only when positioned and pressed against the skin, facilitating a consistent puncture depth for easier sampling.

Improved visibility
The lancet covers only a small area at the contact point, which improves the visibility of the desired puncture site.

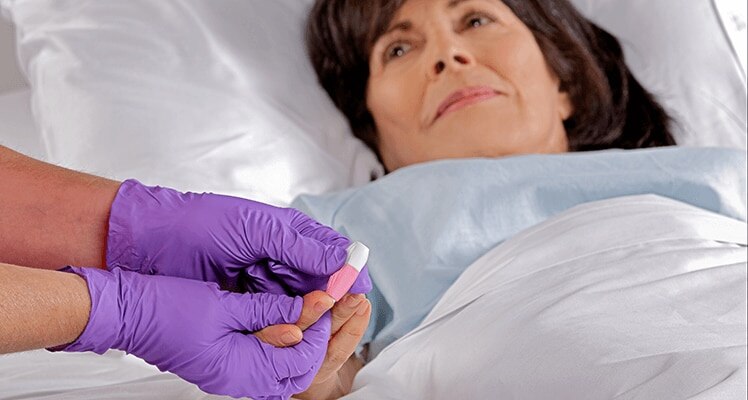
Intuitive procedure
The intuitive procedure reduces training time, and the design prevents product reuse, reducing the possibility of patient, clinician, and/or sample contamination.
false
true
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Notes
* Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS): Procedures and Devices for the Collection of Diagnostic Blood Specimens by Skin Puncture; Approved Standard (Sixth Edition). Wayne, PA, USA, 2008: Document H4-A6.
This medical device is CE marked in compliance with European Medical Device Directive 93/42/EEC and is CE certified by NSAI - National Standards Authority of Ireland (Notify Body Number = 0050 for BD Microtainer® Contact-Activated Lancet (CAL) and BD Microtainer® Quikheel™ Lancet)
BD-18634
true


