RELATED PRODUCTS NOT AVAILABLE
true
BD Kiestra™ Imaging applications for urine and MRSA cultures
AI that enables high resolution and standardised plate imaging


- Overview
- Product Attributes
- Products & Accessories
- EIFU & Resources
CE –IVDR applications developed with Artificial Intelligence (AI) that support urine and MRSA culture reading and interpretation.
Powered by BD Synapsys™ informatics solution, the BD Kiestra™ Urine Culture application (UCA) and Methicillin-resistant Staphylococcus aureus application (MRSA), are designed to support workflow efficiency and enhances quality by providing standardised, diagnostically relevant images for interpretation by laboratory staff.

Drive standardisation
In conjunction with the BD Kiestra™ ReadA Smart incubator, the BD Kiestra™ Optis imaging technology and the BD Kiestra™ imaging applications, benefit from true-to-life imaging, powered by AI-trained algorithm software to standardise your culture interpretation.
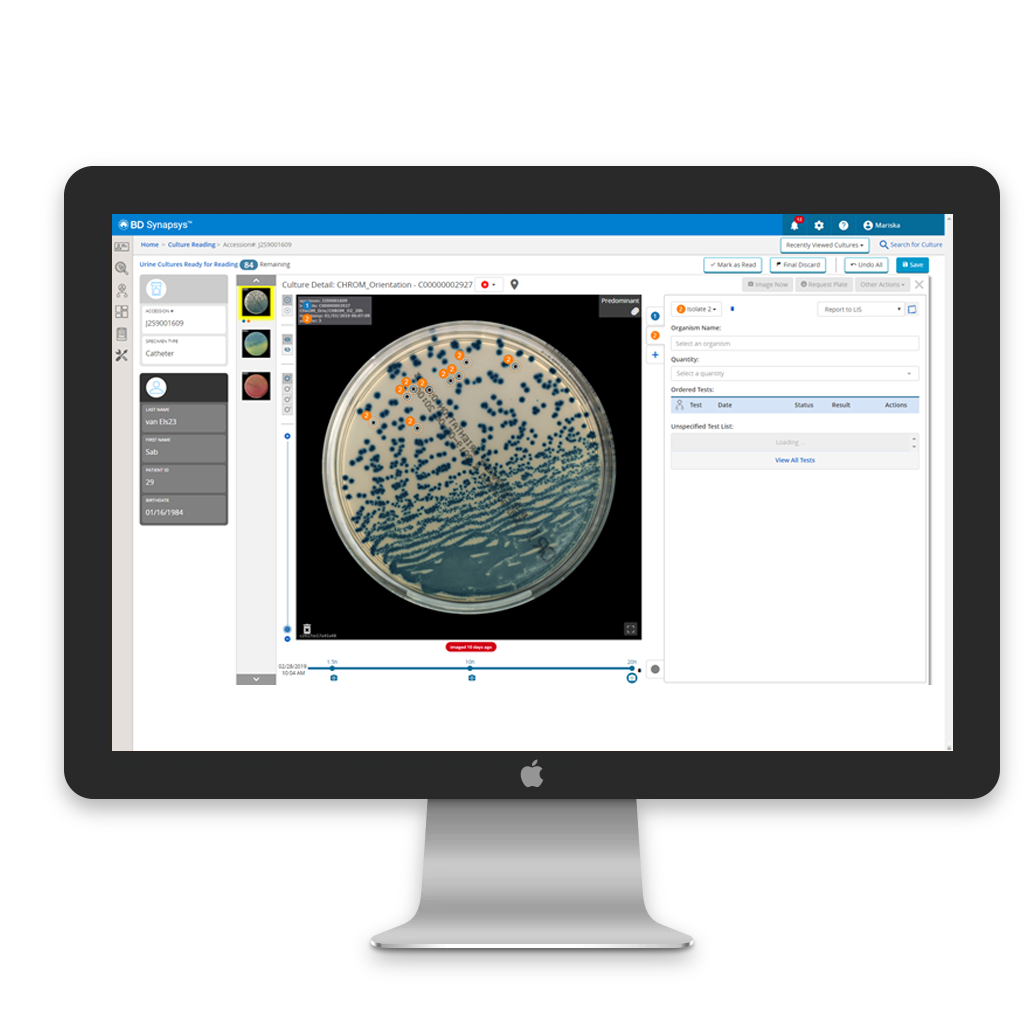
Support workflow efficiency
BD Synapsys™, our informatics solution, offers a customisable framework for timely and organised reading through user-defined worklists.
In combination with BD Kiestra™ Imaging applications, it provides valuable insights to expedite work: accurate growth/no growth detection, quantification of growth levels, color detection, and purity score definition.
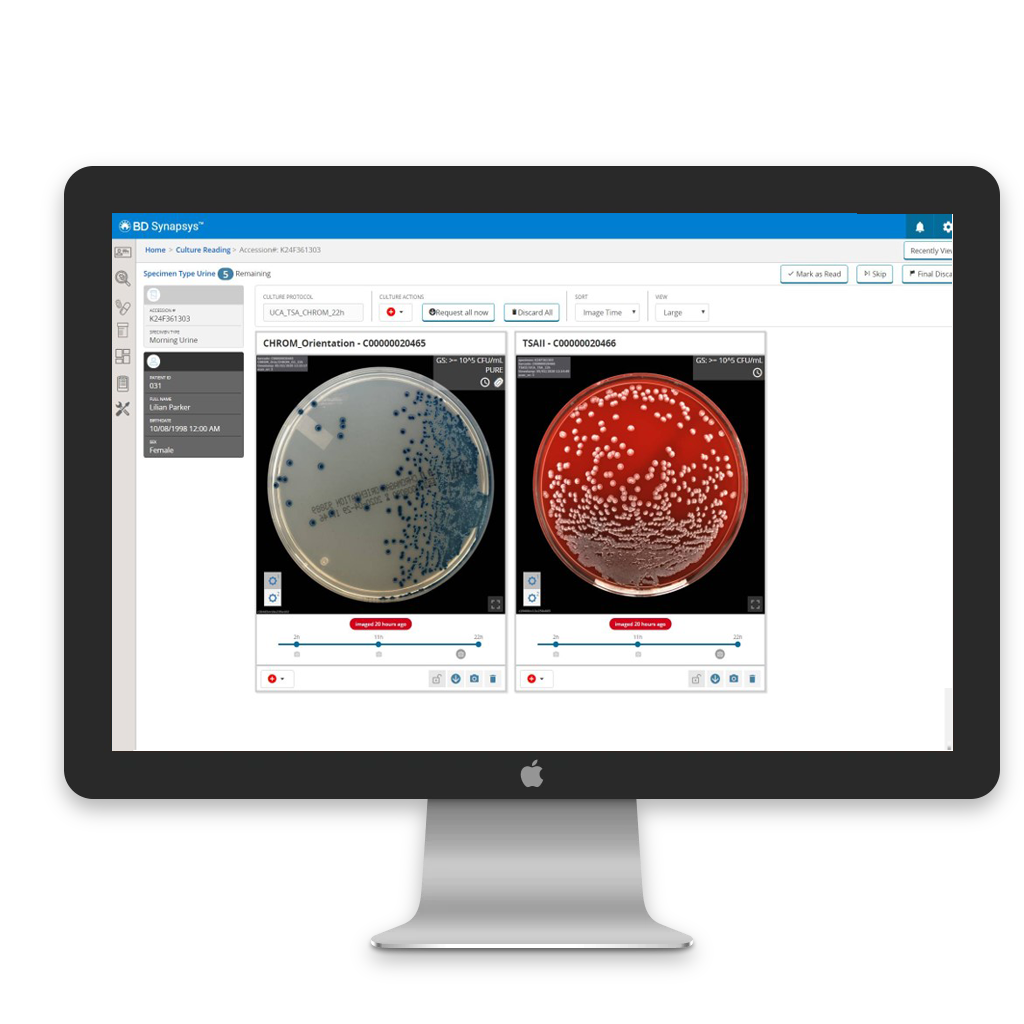
Maximise laboratory staff value
Negative cultures can be reviewed in batches by laboratory staff or automatically released by the BD Kiestra™ Imaging applications, saving valuable time and eliminating unnecessary tasks. This allows your staff to concentrate on tasks where their expertise truly matters.
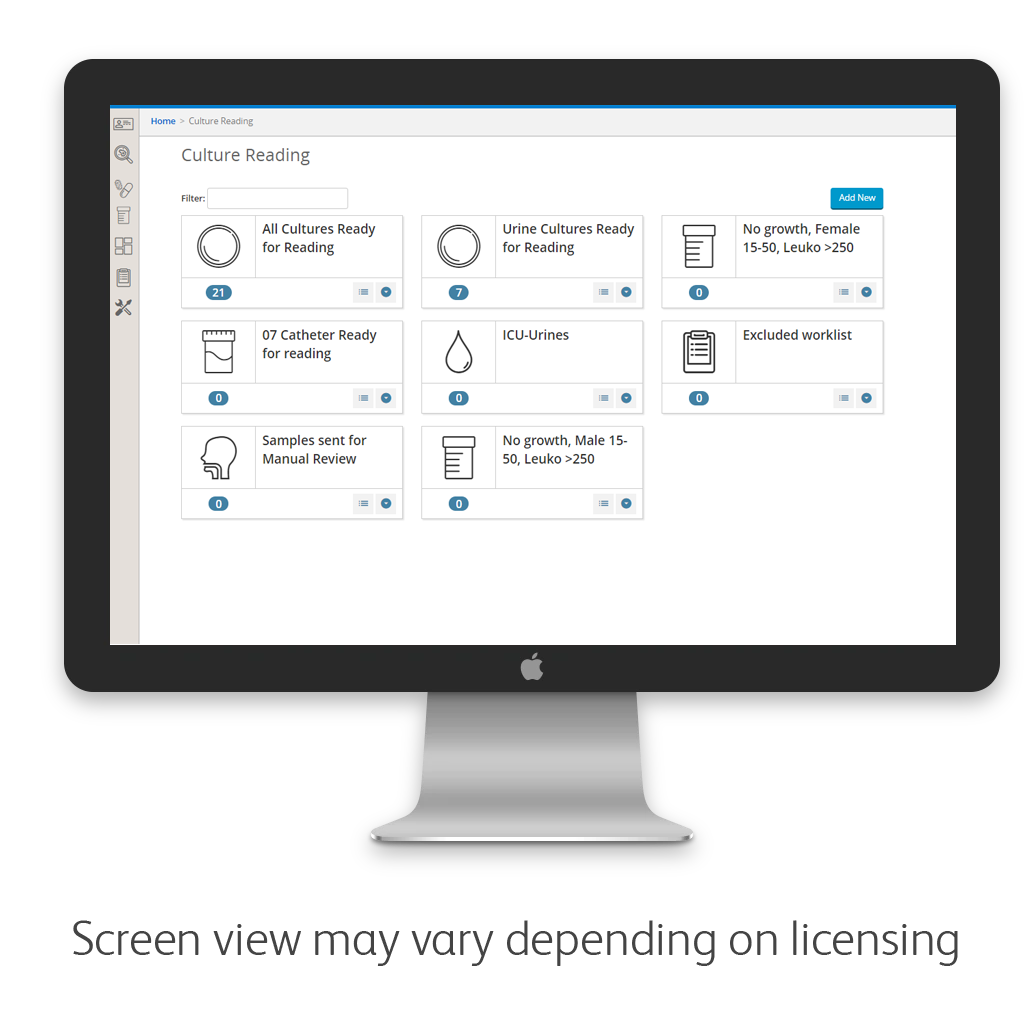
Workflow management
BD Synapsys™ informatics solution enables laboratories to address workflow challenges, including streamlining culture reading processes for timely reporting of critical results while managing workload to improve operational effectiveness.
Closed door incubation
Available as part of a track-based BD Kiestra™ laboratory automation solution or with the BD Kiestra™ ReadA™ standalone systems.
True-to-life and timely imaging
The system features a 25MP camera equipped with a telescoping lens to minimise imaging aberrations. Leveraging BD Kiestra™ Optis technology, it generates standardised images by selecting the best pixels from 22 images on the same plate, ensuring consistent culture reading. Additionally, three light sources optimise colony presentation for accurate analysis.
Culture triage
BD Kiestra™ Imaging applications allows growth and no growth detection to organise your reading work and expedite negative cultures reporting.
Negative culture release
Based on user expert rules, BD Kiestra™ imaging app can automatically release negative cultures (sterile or with non-significative growth), as validated in the applications IFU.
Positive culture review workflow
Powered by BD Synapsys™, positive cultures requiring review can be efficiently sorted into user-defined and insightful worklists. These worklists serve multiple purposes: organisation, ensuring efficient workflow management, and effective workload management.
Example : Pure E.coli cultures, mixed urine cultures, MRSA cultures with mauve detection
Cultures interpretation
Urine cultures :
Our system provides growth detection and assigns a growth score to urine cultures.
When used with a chromogenic plate, the application offers colour detection, along with a presumptive ID and purity score (pure, predominant, or mixed).
MRSA Cultures :
Our system categorises plates into three worklists: No Growth, Growth Without Mauve Colony Growth with Mauve Colony(ies)
Validated media
The validated media types to be used for automated growth evaluation with plated urine cultures
BD BBL™ Trypticase™, Soy Agar II with 5% Sheep Blood
BD BBL™, CHROMagar™ Orientation
BD BBL™, Trypticase™ Soy Agar II with 5% Sheep Blood/BD BBL™ CHROMagar™ Orientation
BD BBL™ Trypticase™ Soy Agar II with 5% Sheep Blood/BD BBL™ MacConkey II agar
BD BBL™ MacConkey II agar
Footnotes
*Croxatto, A., Dijkstra, K., Prod’hom, G. & Greub, G., (2015). Comparison of Inoculation with the InoqulA and WASP Automated Systems with Manual Inoculation. Journal of Clinical Microbiology, vol. 53 (7), 2298 – 2307.
The BD Kiestra™ Urine Culture Application (UCA) powered by the BD Synapsys™ Informatics Solution and the BD Kiestra™ Methicillin-resistant Staphylococcus aureus (MRSA) Application powered by the BD Synapsys™ Informatics Solution are in vitro diagnostic medical devices bearing a CE mark, approved by BSI Group, The Netherlands B.V. (Notified Body no: 2797)
BD BBL™ CHROMagar™, BD BBL™ MacConkey II™, BD BBL™ Cled and BD BBL™ Columbia PPMs are in vitro diagnostic medical devices bearing a CE mark.
CHROMagar is a trademark created by Dr. A. Rambach and is the property of Company BALEL.
false
true
true